The Relevance of the Urine Sediment Exam in Diagnosing Kidney Diseases
AJKD’s freely available Core Curricula aim to give nephrology trainees a strong knowledge base in core topics. The first Core Curriculum topic for 2019 is the examination of the urine sediment by Corey Cavanaugh and Mark Perazella. AJKD Social Media Editor, Timothy Yau (AJKDBlog), interviewed Dr. Cavanaugh (CC) to highlight some of the important teaching points in this Core Curriculum.
AJKDBlog: Let’s start with the tough question: Many residents and nephrology fellows don’t perform independent examination of urine sediment, either because of time constraints, lack of education, or because automated tools may be sufficient. Check out this Twitter Thread by @Nephro_Sparks:
“… examination of urine sediment by nephrologists a relatively rare event”
Just not understanding this statement. I (and every single Nephrologist I know) spins urine on every patient with AKI and many in clinic with CKD if indicated. On a given day several times. Others?? https://t.co/fqTKDSpJDW
— Matthew Sparks, MD (@Nephro_Sparks) September 26, 2018
Why do you think it’s important to continue to emphasizing spinning urine as a core skill for a nephrologist?
CC: For now, urine sediment is still the most accurate, timely, and inexpensive biomarker we have for AKI and other forms of kidney disease. Many trainees will start the AKI work-up by calculating the fractional excretion of sodium (FENa) and maybe give a bolus or continuous infusion of intravenous fluids. As FENA can be <1% in various causes of acute tubular injury, this would be the wrong approach, which could be potentially harmful to the patient. On the other hand, if you start with analysis of the urine, you realize it’s much more heterogenous than pre-renal vs ATN. There may be evidence for acute tubular injury [renal tubular epithelial cells (RTEC) casts, and granular casts], glomerular bleeding, and white blood cell casts, in real time. I think it requires the nephrologist to expand their differential and really think about the case as you analyze the urine. In fact, the urine sediment examination can change both diagnosis and management of many patients. Also, I think it’s just so cool to see the faces of fellows, house staff, and students light up when they find that muddy brown cast or acanthocyte they always read about. It becomes their diagnosis.
AJKDBlog: For those trainees who are relying on automated technology for urine examination, what are the big differences between manual and automated urine microscopy? What are the major pros and cons for each?
I think with only automated microscopy you’re likely missing a lot of important sediment findings. It is insensitive to RTECs, pathologic casts, and crystals as we’ve outlined in the paper. Although manual microscopy takes time to collect, analyze, and it’s not a billable item, you can (and should) analyze the urine on multiple days in a row, in detail, to get good insight into the process within the kidneys. You may see something totally different from day 1 to day 5. Depending on the findings, choices concerning intravenous fluid administration, stopping crystal or AIN-producing antibiotics, and kidney biopsy can be undertaken. Seriously, when is the last time you saw your lab report state ‘crystalluria likely from acyclovir’? With automated microscopy, yes, it’s quicker and less labor intensive, and there are images that can be obtained and reviewed by technicians. However, it remains less accurate than the trained nephrologist’s eye that is analyzing the spun urine manually. Additionally, it gives educators a chance to slow down and use the time while spinning the urine and viewing the sediment to teach and discuss nephrology and urine sediment.
One of the hurdles is the CLIA (Clinical Laboratory Improvement Amendments) laws which prohibit urine microscopy outside of the hospital’s central laboratory. At Yale-New Haven Hospital, nephrology faculty and fellows are able centrifuge the urine and view the sediment outside the central lab by having Provider Performed Microscopy certification granted by the state (fee and didactic training required).
Urine sediment shows (A) renal tubular epithelial cells (RTECs) with a single nucleus and (B) RTEC cast, with multiple RTECs within the cast matrix. Figure 2 from Cavanaugh and Perazella, AJKD, © National Kidney Foundation.

Granular or muddy brown casts of various widths and lengths are seen in a patient with acute kidney injury due to septic shock. Figure 3 from Cavanaugh and Perazella, AJKD, © National Kidney Foundation.
AJKDBlog: Different practitioners tend to spin their urine in their own unique way, be it volume, time, RPMs, etc. In the Core Curriculum, you recommend a standardized approach. Can you review it here, and tell us why you think this is the best way to perform a urine sediment exam?
CC: We recognize that spinning urine is time-consuming (although 5-10 minutes do not seem like a lot), and when compounded multiple times during a busy day it can be taxing. It is true that many different practitioners have been trained to spin at different speeds and times in different centrifuges. Investigators have utilized different parameters in their evaluations of urine microscopy scoring. In their 2008 study of AKI and cast scoring index, Chawla et al centrifuged 10 mL of urine at 2,000 rpm for 10 minutes. In a 2012 study by Bagshaw et al, they evaluated urine microscopy in AKI and spun urine at 1,500 rpm (approximately 225 x g) for 10 minutes. Perhaps the greatest microscopist of our time, Dr. Giovanni Fogazzi, recommends spinning 10 mL of urine for 10 minutes at 400 x g.
Ultimately, it depends on your centrifuge, as the g-forces generated depend on the radial arm’s length of the centrifuge. Most centrifuges are ~14-15 cm, so if you were to spin at 1,500 rpm, the relative centrifugal force (g) would be approximately 350. European guidelines recommend 400 x g for maximum yield and beyond this, you are likely to cause cell wall rupture. Whatever methodology is applied, whether you pipette or manually agitate, whether you look at your slide for 5 minutes or 15, perhaps the most important part is standardization for repeatability. We emphasize this above all else.
AJKDBlog: In the Core Curriculum, you go over several types of cellular casts and the associated kidney diseases. Almost every med student knows to think about acute interstitial nephritis when they hear “WBC casts” and acute tubular necrosis when they hear “Granular casts.” But there are a lot of red herrings that we can see when we spin urine as well. Calcium oxalate crystals, hyaline casts, etc can be seen in healthy individuals. Can you share some of the major limitations of the urine sediment exam?

A hyaline cast is noted in a patient with acute kidney injury in the setting of decompensated heart failure. Figure 4 from Cavanaugh and Perazella, AJKD, © National Kidney Foundation.
CC: Indeed, there are limitations to urine microscopy. We call it the ‘liquid biopsy’, but we need to remember it is not meant to be a replacement of a tissue diagnosis. There are certainly cases in which RBC casts have been identified by urine microscopy; however, a tissue diagnosis ultimately revealed acute interstitial nephritis. The presence of crystals such as uric acid and calcium oxalate does not always indicate that these crystals are causing the underlying kidney injury. They can be completely unrelated to the kidney injury. RTEC casts and granular casts are most commonly seen in the setting of acute tubular injury; however, these casts may also be seen with acute interstitial nephritis, thrombotic microangiopathy, vasculitis, and more.
Additionally, the presence of RTEC or granular casts with acute tubular injury doesn’t tell the clinician why the tubules were injured. Was it a nephrotoxic drug, hypotension, rhabdomyolysis, or another form of acute tubular injury? As with any diagnostic test, urine microscopy requires and depends on the nephrologist’s clinical acumen to be used appropriately.
AJKDBlog: Kind of a weird question to end with here. There are several great images of different casts and crystals included in the Core Curriculum. Which one is your favorite, and why?
CC: Tough question! I am partial to the acanthocyte. They can be difficult to spot, but when found can completely change the trajectory of the case. What GN could they have? Should we biopsy? Nothing could be more exciting for me!

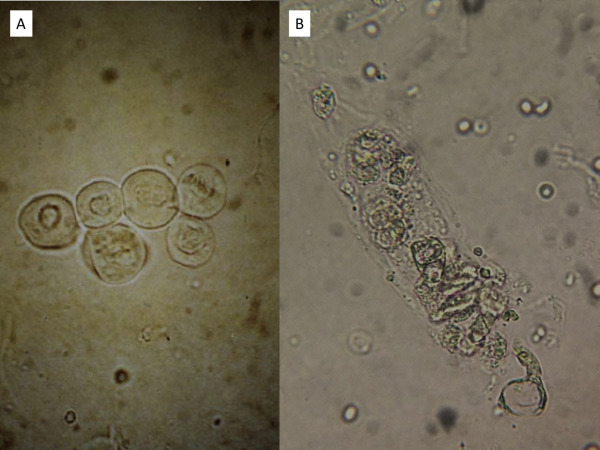
Urine sediment of a patient with infection-related glomerulonephritis reveals (A) dysmorphic red blood cells (RBCs), including acanthocytes and isomorphic RBCs, and (B) RBC cast. Figure 5 from Cavanaugh and Perazella, AJKD, © National Kidney Foundation.
AJKDBlog: Thank you for taking the time to do this interview!
Follow Dr. Cavanaugh @ccavanaugh87
Title: Urine Sediment Examination in the Diagnosis and Management of Kidney Disease: Core Curriculum 2019 (freely available)
Authors: Corey Cavanaugh and Mark A. Perazella
DOI: 10.1053/j.ajkd.2018.07.012

Leave a Reply