Understanding the intersection of the biology of pain, opioid therapy, and disease progression and survival is complicated and potentially of great importance in cancer care. Pain occurs in two-thirds of patients with advanced malignancy and continues to impact a third of patients with cancer even after completion of curative treatment.[61] Pain and other symptoms affect quality of life (QoL) during and after treatment.[5,43,48,59] In addition, pain itself has been associated with shorter survival in diverse malignancies.[72] As long as the benefits clearly outweigh risks, and opioids are safe and effective for cancer-related pain, this therapy is an important option.[9,46] Like all medical drugs, however, opioids have unavoidable adverse effects. These include constipation, nausea, sedation, cardiovascular instability, respiratory depression, central nervous system toxicity, hyperalgesia, tolerance, and opioid use disorder.[46] Such risks are well understood and typically discussed with patients. On the other hand, there is less awareness about the potential adverse effects of pain and opioids on the growth of certain cancers. Here, we review the evidence regarding opioid therapy, disease progression, and survival to raise awareness and stimulate greater dialogue regarding these issues among stakeholders in the cancer and pain communities. By doing so, our goal is that patients with cancer will be better positioned to address their coexisting priorities of pain relief and survival.
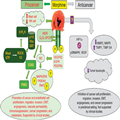
The analgesic activity of opioids is mediated through mu-opioid receptors (MORs) in the central nervous system. Mu-opioid receptors are also present on endothelial cells[33] and in human tumors (peripheral MORs), including prostate and lung cancer.[22,39,40,52] Preclinical studies show that opioids promote angiogenesis by stimulating endothelial proliferation and migration and by activating survival- and growth-promoting signaling through protein kinase B (Akt) and mitogen-activated protein kinase pathways, respectively, in the endothelium (Figure 1).[16,24,36] In addition to promoting tumor angiogenesis, chronic morphine treatment also stimulates lymphangiogenesis, activates mast cells, promotes tumor growth and metastasis, impairs survival in mouse models of breast cancer,[2,33,45] and is immunosuppressive.[47,62] Furthermore, MORs and receptor tyrosine kinases are expressed and colocalized in advanced lung cancer, which may play a role in cancer growth and spread.[39,53] Preclinical studies provide strong evidence that in animal models of several different malignancies, activation of peripheral MORs (on endothelial cells and tumors) by clinically used opioid medications promotes tumor progression through several different mechanisms. These involve signal transducers and activators of transcription 3 (STAT3), mitogen-activated protein kinase/extracellular signal–regulated kinase and Akt signaling pathways, nitric oxide synthesis, cyclooxygenase (COX)-2 activation, prostaglandin E2 production, cross-activation of epidermal growth factor receptor and vascular endothelial growth factor receptor 2 (VEGFR2), release of substance P, and mast cell activation.[8,13,24,27,33,36,40] Our recent findings on morphine-induced retinal neovascularization in mice with sickle cell disease further validate the role of morphine in promoting angiogenesis through coactivation of VEGFR2 and the contribution of inflammatory cytokines and the STAT3 pathway in stimulating expression of endothelial MOR.[24] Heightened inflammation as well as activation of VEGFR2 and STAT3 signaling are the rule, not the exception, in most cancers. Importantly, opioids through MORs contribute to epithelial mesenchymal transformation in lung cancer, a process critical for progression of this cancer.[35] Recent clinical studies raise the possibility that these mechanisms may play a role in both cancer progression and nociception in patients. These mechanistic insights also provide targets for intervention to ameliorate the inadvertent effect of opioids on cancer progression and QoL (Figure 1).
Figure 1.
Mechanisms of opioid activity in cancer. Preclinical and clinical studies demonstrate multiple signaling pathways and cellular effects stimulated by morphine and/or MOR, leading to progression of cancer and metastasis (left side). MOR directly and through coactivation of receptor tyrosine kinases for growth factors, VEGFR2, EGFR, and PDGFRβ stimulates mitogenic and survival-promoting signaling through MAPK/ERK, Stat3, and PKB/Akt in endothelial and/or tumor cells. Simultaneously, morphine activates S1P3R through Rho/ROCK pathway after the recruitment of p115 Rho GEF by MOR, leading to increased vascular permeability. Inhibition of NK cells and activation of mast cells by morphine further abrogates protective antitumor effects and simultaneous release of procancer cytokines and neuropeptides such as substance P, respectively. In addition, stimulation of COX2 leads to formation of PGE2, which has proangiogenic and pronociceptive activity and thus may even increase pain. Together, morphine/opioid-induced cellular effects and signaling pathways lead to endothelial and tumor cell proliferation, migration, invasion and EMT, immunosuppression, and increased vascular permeability, which is critical to tumor cell infiltration and metastasis, thus promoting cancer progression and metastasis. Although most of the strong evidence is from human tumor and endothelial cells and mouse models of cancer and metastasis, there are emerging data from clinical studies (mostly retrospective) showing the association of MOR with these signaling pathways and/or cellular activation in lung, prostate, and pancreatic cancer, leading to cancer progression and shorter survival. Conversely, antitumor effects of morphine/opioids through modulation of HIF1α, p38 MAPK, VEGF, MMPs, and TIMPs in endothelial and/or tumor cells lead to inhibition of cancer progression in mice. However, the only study in a clinical setting failed to replicate the preclinical observations on MMPs. COX2, cyclooxygenase 2; EGFR, epidermal growth factor receptor; ERK, extracellular signal–regulated kinase; GEF, guanine nucleotide exchange factor; GPCR, G-protein–coupled receptor; HIF1α, hypoxia inducible factor 1 α; MAPK, mitogen-activated protein kinase; MOR, mu-opioid receptor; NK cell, natural killer cell; NO, nitric oxide; PDGFR, platelet-derived growth factor; PGE2, prostaglandin E2; ROCK, rho-associated protein kinase; RTK, receptor tyrosine kinase; S1P3R, sphingosine 1 phosphate receptor 3; Stat3, signal transducer and activator of transcription 3; TIMP, tissue inhibitor of metalloprotease; VEGF, vascular endothelial growth factor; VEGFR2, vascular endothelial growth factor receptor 2.
Pain also increases circulating endogenous opioid (eg, endorphin) levels and causes significant immunosuppression.[47,62] Therefore, it is possible that even in patients who are not receiving opioid medications, elevated levels of endogenous opioids may activate peripheral MORs and cross-activate signaling pathways that influence tumor progression. Patients with inadequate pain control after major surgery also develop significant immunosuppression.[62] These mechanisms may contribute to the known adverse prognostic effect of pain in malignancies.[43,50,59,72]
Several lines of evidence suggest that pain, systemic opioid exposure, and opioid receptor activity may be associated with adverse cancer outcomes in humans. In early-stage cancers (primarily those treated with potentially curative surgery), some retrospective studies reported that exposure to systemic opioids during anesthesia for cancer surgery was associated with a higher incidence of recurrence and metastases,[14] but other studies noted no such association.[6] Opioid reduction strategies with multimodal analgesia have been investigated as a potential means to reduce cancer recurrence. These strategies included administration of nonsteroidal anti-inflammatory drugs or COX-2 inhibitors (eg, celecoxib), which are known to have significant anticancer effects.[17,18] Celecoxib slightly reduced perioperative cyclooxygenase activity during cancer surgery and lower postoperative pain scores.[29] Complementary to these observations, celecoxib ameliorated the proangiogenic effect of morphine in a breast cancer model while reducing hyperalgesia compared with morphine.[16] Dexmedetomidine has been successfully included in protocols designed to reduce perioperative opioid consumption. Two recent investigations in animals and humans have indicated a potential negative impact of dexmedetomidine in the context of cancer.[7,34] Yet, this drug has been shown to induce apoptosis in oral squamous carcinoma and glioblastoma cells and to have significant anti-inflammatory effects when given in cancer surgery, which could potentially offset the negative effects of inflammation in cancer progression.[13,60] Ketamine and gabapentinoids can affect survival, proliferation and metastatic activities in cancer cells, and modulation of the immune system. Although there is no evidence to recommend the use of any of these adjuvant analgesics to reduce cancer after oncologic surgery or any other clinical setting,[42] further research has been recommended.[29] In addition, it has been hypothesized that reduction of opioid consumption by implementation of regional anesthesia during oncologic surgery would be associated with lower rates of cancer recurrence or longer recurrence-free survival.[42] After an initial report in 2006 that use of regional anesthesia was associated with a significant decrease in breast cancer recurrence after mastectomy,[15] more than 20 retrospective studies have reported conflicting results. Accordingly, a recent consensus article by the American and European Societies of Regional Anesthesia concluded that there is insufficient evidence to recommend regional anesthesia to reduce cancer recurrence.[42]
In patients with advanced, metastatic cancers, retrospective studies reported that higher MOR expression is associated with poorer clinical outcomes in advanced cancers of the prostate,[70] lung,[39] stomach,[67] and esophagus,[69] and that impaired opioid receptor activity is associated with longer survival in breast cancer.[4] In the first study to simultaneously examine the association of quantitative long-term opioid use and level of MOR expression with cancer outcomes, we reported that higher MOR expression and greater opioid requirement are independently associated with shorter progression-free and overall survival in patients with metastatic prostate cancer receiving first-line androgen deprivation therapy.[70] We also found that chronic pain and greater opioid requirement early after diagnosis are independently associated with shorter survival in advanced non–small cell lung cancer.[71] However, the retrospective clinical studies had limitations, including some that used analgesic intake as an indicator for cancer pain, thus making it difficult to determine whether pain or opioid therapy was more relevant in impacting survival. Some prospective studies indicate that reducing systemic opioid exposure may improve survival in advanced cancer; eg, by celiac plexus block in pancreatic cancer[38] and administration of opioids through intrathecal pump in various malignancies.[55] Retrospective studies also reported that pain is independently associated with shorter survival in metastatic prostate,[1,10,11,25,56] lung,[28] and various other malignancies.[72]
By contrast, other basic and preclinical studies suggest a potentially protective effect of certain opioids in cancers. For example, methadone increases sensitivity of some cancer cell lines to chemotherapy. Methadone is a racemic mixture of the 2 enantiomeric forms, d-methadone and l-methadone, with the l form having 10 times more affinity to MOR than the d form. It is a unique opioid with agonist activity at the mu and delta opioid receptors and antagonist activity at the N-methyl-D-aspartate receptor.[58] Methadone seems to have an anticancer effect by activating apoptosis pathways by caspase activation and downregulation of X-linked apoptosis protein and B-cell lymphoma-extra-large.[19] Methadone also overcomes apoptosis resistance and chemotherapy resistance in leukemic cells by activating apoptosis pathways,[20] and may sensitize glioma cells to doxorubicin.[19] Other in vitro experiments showed that high concentrations of MOR agonists such as morphine (1–10 μM) and D, L-methadone (1–10 μg/mL) had direct proapoptotic effects on gastric cancer, leukemia, and glioblastoma cells.[19,21,49,57] Similar effects were observed in animals: Chronic treatment with fentanyl (>14 days, 0.4 mg/kg, twice the analgesic dose) suppressed tumor growth in mice implanted with a gastric cancer cell line.[27] In addition, very high doses of morphine (30 vs 0.5–5 mg/kg) induced tumor shrinkage through antiangiogenesis in a mouse model of lung cancer.[32] Also, in vitro and in vivo studies have shown that MOR agonists inhibited metastatic mechanisms in breast and colon cancer cells. Some antimetastatic effects were linked to inhibition in production and release of matrix metalloproteinase-2 and -9 and adhesion molecules including intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin.[23,26,27,41,65] However, in a clinical setting, there was no correlation between perioperative morphine intake and MOR activation or matrix metalloproteinases.[66] Furthermore, although many patients with advanced cancers receive high doses of opioids for long periods, opioids have not been reported to be associated with tumor regression or inhibition of metastasis.
Another line of research has targeted MORs for anticancer therapy. Preclinical studies demonstrated that MOR antagonists inhibited tumorigenic and metastatic behaviors in colorectal and lung cancer cells and synergistically potentiated the antiangiogenic effects of chemotherapeutic agents such as 5-fluorouracil, bevacizumab, and mammalian target of rapamycin inhibitors through protein tyrosine phosphatase receptor signaling.[37,51,52,64] In animals, low doses of naltrexone enhanced the anticancer effects of cisplatin in a mouse model of ovarian cancer.[12] In vivo studies are in agreement with the antitumoral effects of MOR antagonists not only in cancer cells but also in cells of the tumor microenvironment. As an example, the MOR antagonist CTOP (D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2) reverses the immune-suppressive effects of opioids.[3] One clinical study has reported (in a post hoc analysis) the association between the administration of a MOR antagonist, methylnaltrexone, and overall survival of patients with advanced malignancies. This study included patients from 2 randomized controlled trials designed to assess the efficacy of methylnaltrexone vs placebo for treatment of opioid-induced constipation. Methylnaltrexone administration was associated with significantly longer overall survival in patients with cancer, but not in patients without cancer. This finding suggests that the beneficial effect of opioids on overall survival may be specific to cancer pathogenesis.[30]
In summary, although preclinical evidence linking opioids to cancer growth appears robust, whether and how this phenomenon may be occurring in humans requires further investigation. What may be true in theory or in laboratory and animal studies may be quite different from phenomena occurring in humans. Furthermore, most of the preclinical data are for morphine, whereas other opioids such as hydromorphone, methadone, or fentanyl, which are often used to treat pain in advanced malignancies, have not been investigated as extensively. Taken as a whole, the current status of understanding of adverse effects of pain and opioid therapy on tumor progression and overall survival is complicated and not definitive. In fact, we do not even have the first series of clinical trials on opioids and disease progression to guide us. In existing studies, it is difficult to determine the specific contribution of opioids to overall results and outcomes. This is an important area for future research.
Current awareness of the opioid epidemic has led to initiatives aimed at discovering nonopioid analgesics. However, many recent nonpharmacologic strategies such as deep brain stimulation and spinal cord stimulation function by releasing endogenous opioids. Therefore, the roles of pharmacologic as well as endogenous opioids need to be investigated in prospective clinical studies, to identify their inadvertent effects and develop specific preventive strategies (Figure 2).
Figure 2.
Proposed mechanism-based interventions to ameliorate opioid-induced cancer progression.
Taken together, these findings lead potentially to both a practical and ethical predicament. It is not conscionable to undertreat pain or to not consider existing research on potential adverse effects of opioid therapy on cancer progression and overall survival. A delicate balance needs to be accomplished between treating pain with opioids to improve functional status and tolerability of cancer treatment while preventing overprescribing of opioids which may cause undesirable side effects and potentially affect cancer outcomes. Providers will need to remain updated about the rapidly evolving evidence base regarding the potential adverse effects of pain or opioid therapy in patients with advanced malignancies.
It is understandable that the lack of definitive findings about the adverse roles of pain and opioid therapy may be challenging for patients and physicians when deciding on pain management strategies. Given the primarily preclinical and emerging clinical evidence base regarding opioids, pain, and survival, it may be premature to discuss such information in great detail when obtaining initial informed consent for pain treatment. Reduction of patient suffering remains the foremost treatment goal, together with the ethical obligation to provide sufficiently informed, patient-centered care. When safe and appropriate, providers can recommend the use of nonopioid therapies and other pain management interventions, and use opioids sparingly and for a short period, in early-stage curable cancer where pain is usually due to cancer treatment or related to noncancer conditions.[46,48] On the other hand, patients with advanced cancer, who experience pain related to progressive cancer, may be enrolled in studies testing strategies to reduce the potential adverse impact of long-term opioids on tumor progression. One such ongoing study (ClinicalTrials.gov #03087708) is the first prospective, multicenter, randomized, placebo-controlled, double-blind study testing whether a peripheral opioid receptor antagonist improves QoL and cancer outcomes in patients with advanced non–small cell lung cancer.
It is our hope that the relevant safety and ethical obligations of pain management and opioid therapy among patients with cancer will be openly and transparently discussed among the diverse stakeholders, including patients, health care providers, regulatory and governmental agencies, pharmaceutical industry, and other interest groups. It is also our hope that prospective studies will investigate the efficacy of opioid-sparing strategies and novel nonopioid analgesics. We recommend that opioid use be recorded for all patients with cancer, including (1) the etiology of pain for which opioids are required (ie, cancer vs noncancer pain,[46,48] (2) the specific opioids and doses taken, (3) whether opioids were taken before a diagnosis of malignancy and, if so, for how long and for what condition, and (4) the doses, effectiveness, and tolerability of opioids taken on an ongoing basis after diagnosis of cancer. Such a registry would contain data generated during the course of active cancer treatment and survivorship and could provide information about opioid use and tumor growth. Registry data have informed acute[68] and chronic fields of pain medicine.[31,44,54,63] By doing the same in cancer pain, we hope there will be improved understanding of the roles of pain and opioid therapy on tumor progression and overall survival. Until such prospective data are available, opioids should continue to be used as needed to control cancer pain adequately.
Acknowledgements
Dr P. Gupta received support from the Veterans Health Administration; Dr K. Gupta is thankful to NIH/NHLBI 1U01 HL117664-01 for funding. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Pain. 2020;16(3):496-501. © 2020 International Association for the Study of Pain